Real world evidence from more than 144,000 people with diabetes
Clinically significant glucose improvements seen in real world evidence of patients using the OneTouch Reveal® app and the OneTouch Verio Flex® meter at 6 months*.
Methods: Anonymized glucose and app analytics were extracted from a server from more than 144,000 people with diabetes (PWDs). Data from their first 14 days using the OneTouch Reveal® app and OneTouch Verio Flex® meter were compared with 14 days prior to 90- and 180-day timepoints using paired within-subject differences.
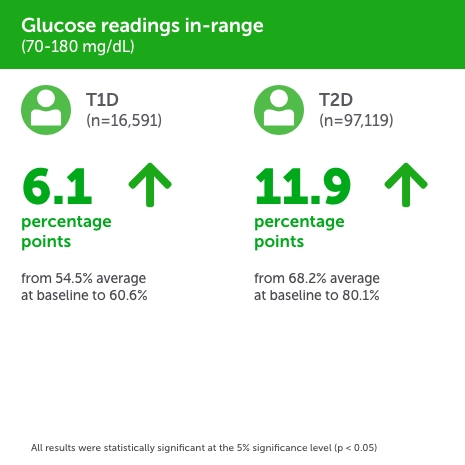
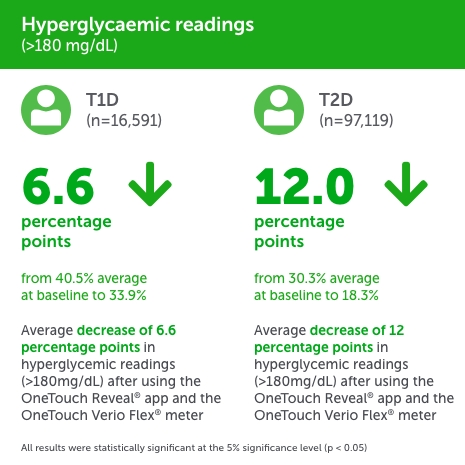
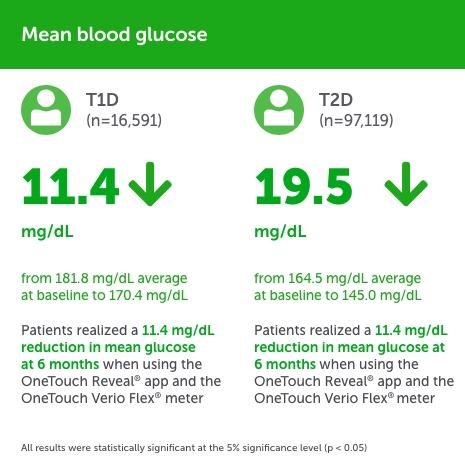
People with type 1 diabetes (PwT1D),
- Readings in-range (RIR) improved by +6.1 (54.5% to 60.6%).
- Hyperglycemia was reduced by −6.6 (40.5% to 33.9%).
- People with type 1 diabetes spending two to four sessions or 10 to 20 minutes per week on the app improved RIR by +5.1 and 7.0, respectively.
- Mean blood glucose (BG) reduced by −11.4 from baseline to 180 days, with no clinically meaningful changes in percentage of hypoglycemic readings. All glycemic changes were statistically significant (P < .0005 level).
People with type 2 diabetes (PwT2D),
- Readings in-range (RIR) improved by +11.9 percentage points (68.2% to 80.1%).
- Hyperglycemia was reduced by −12.0 (30.3% to 18.3%).
- People with type 2 diabetes spending two to four sessions or 10 to 20 minutes per week on the app improved RIR by +11.6 and 12.0, respectively.
- Mean BG reduced by −19.5 mg/dL from baseline to 180 days, with no clinically meaningful changes in percentage of hypoglycemic readings. All glycemic changes were statistically significant (P <. 0005 level).
Real-world data from more than 144 000 PWD demonstrated improved percentage readings in-range and reduced hyperglycemia in people with diabetes using the OneTouch Reveal® app and OneTouch Verio Flex® blood glucose meter.
* Grady M, Cameron H, Holt E. Improved Glycemic Control Using a Bluetooth®-Connected Blood Glucose Meter and a Mobile Diabetes App: Real-World Evidence From Over 144 000 People With Diabetes. Journal of Diabetes Science and Technology.
2023;0(0). doi:10.1177/19322968221148764